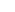PIPELINE
| Classification | PIPELINE | Candidate material | Preclinical | Clinical trial | New Drug approval | Item authorization | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||||
| Cell therapy (*Treg cell) |
Alzhimer's disease |
VT301 | |||||||
|
|||||||||
| Botanical medicine | VT012 (PM012) |
||||||||
|
|||||||||
| *COPD VT014 (PM014) |
Acute upper respiratory infection |
||||||||
|
|||||||||
| *COPD | |||||||||
|
|||||||||
| Depression VT011(PM011) | |||||||||
|
|||||||||
| Alopecia / Depilation VT015 |
|||||||||
|
|||||||||
| Botanical functional material (Health functional food) |
Regulation of blood glucose VT021(PM021) |
||||||||
|
|||||||||
*COPD: chronic obstructive pulmonary disease*Treg: immune-regulatory T cell